OFFERING PATIENTS A CHOICE OF SCREENING METHOD MAY IMPROVE SCREENING RATES3
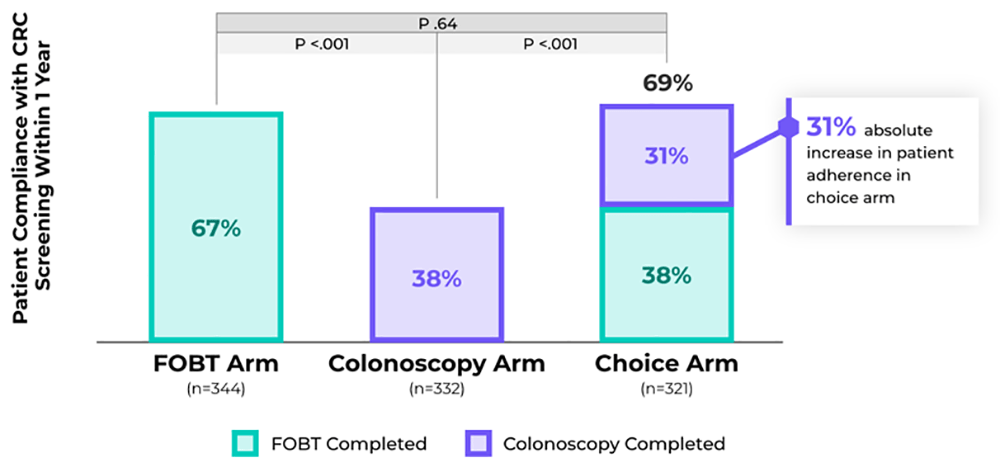
- In a randomized clinical trial of racially/ethnically diverse adults aged 50–79 at average risk of CRC (n=997), health care providers offered patients CRC screening with FOBT, colonoscopy, or patient choice for either, and choice resulted in an absolute increase in patient adherence3
- With increasing trends in CRC incidence (a 1.9% increase per year from 2011 to 2019) and mortality (a 1.2% increase per year from 2004-2020) in adults younger than 50 years,10 it is becoming more critical to detect CRC early
NATIONAL GUIDELINES RECOMMEND SHARED DECISION MAKING TO IMPROVE SCREENING ADHERENCE
| National Guidelines Recommend Shared Decision Making to Improve Screening Adherence | |
|
US Preventive |
“Several recommended screening tests are available. Clinicians and patients may consider a variety of factors in deciding which test may be best for each person” “Discussion with patients may help better identify screening tests that are more likely to be completed by a given individual” |
| American Cancer Society (ACS) 20182 |
“The importance of offering a choice between structural or stool-based testing is included in this guideline in recognition of the role of patient values and preferences and as a practical implementation strategy to improve adherence” |
| American College of Gastroenterology (ACG) 20214 |
“The ‘ideal’ screening test should be noninvasive, have high sensitivity and specificity, be safe, readily available, convenient, and inexpensive. For CRC screening, there are multiple approved tests and strategies, each with its strengths and weaknesses. In some instances, the ‘best’ screening test can be considered the one that is acceptable to the patient and gets completed.” |
References
1 Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977.
2 Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250-281.
3 Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575-582.
4 Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: Colorectal cancer screening 2021. Am J Gastroenterol. 2021;116:458-479.
5 Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterol. 2022;162(1):285-299.
6 Barry MJ, Edgman-Levitan. Shared decision making—pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781.
7 Laiyemo AO, Adebogun AO, Doubeni C, et al. Influence of provider discussion and specific recommendation on colorectal cancer screening uptake among U.S. adults. Prev Med. 2014;67:1-5.
8 American Cancer Society. Colorectal cancer facts & figures 2023-2025. Atlanta: American Cancer Society; 2023.
9 Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: Recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017:112(7):1016-1030.
10 Siegel RL, Miller KD, Wagle NS, et al. Colorectal cancer statistics. CA Cancer J Clin. 2023;73(3):233-254.
Last Updated: 06/04/2023