Ordering your patient’s test is easy

4 simple steps to get your patients tested
Keep your patients screened for cancer. Ordering a Cancerguard test and getting the results is simple and straightforward.
Request blood collection kits
Request blood collection kits

You can request blood collection kits to be shipped to you by calling our customer care team.
Step 2
Place your order
Place your order

Online ordering available starting August 22, 2025
- Our Provider Hub is the most efficient way to submit your order
- If you don’t already have an account, it’s easy to create one
- Once your account is created, it typically takes 24-48 hours to validate your information before you can place your order
Step 3
Collect and ship samples
Collect and ship samples

- The Cancerguard test will require patients to provide 4 blood vials.
- Please ship samples back to us in the supplied Cancerguard blood collection kits, according to our specimen requirements and shipping guidelines.
Step 4
Access results
Access results

- You can access your patients' results on our Provider Hub or receive the report by fax.
- Most Cancerguard test reports are available within two weeks.
Support after a positive result

Exact Sciences provides Care Navigation education and resources to streamline next steps after a positive Cancerguard™ test result, including:
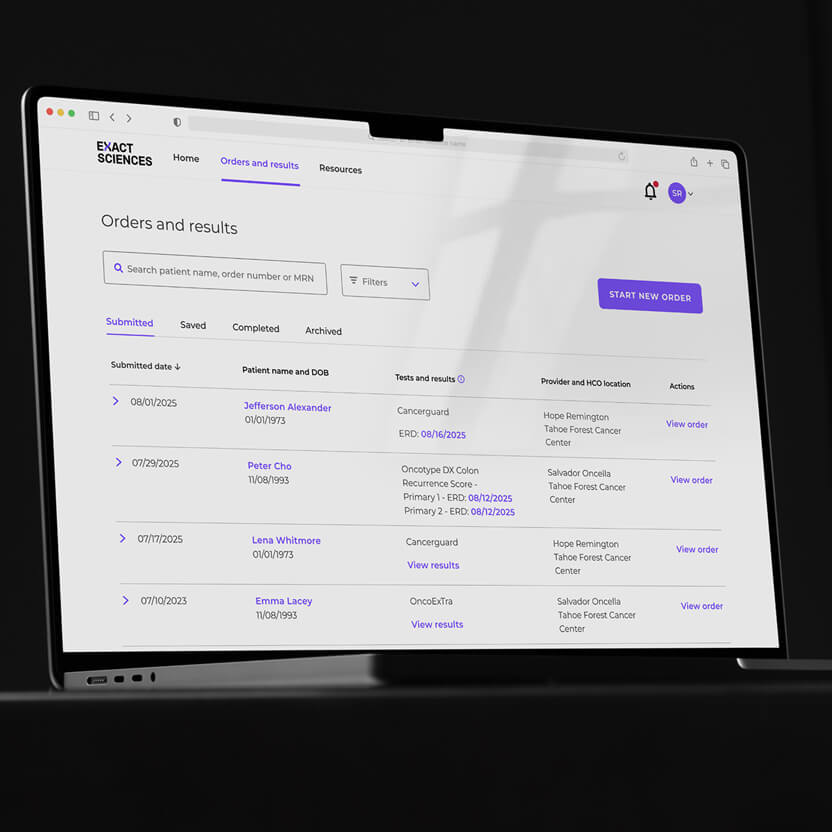
Access everything all in one place
With Exact Sciences' online Provider Hub, ordering a test, tracking test progress, and accessing results is convenient and efficient.
- Orders can be submitted online — virtually eliminating the need for follow-up calls to complete any missing information.
- All patient orders and results can be viewed, tracked, and sorted quickly.
- Orders and results may be viewed online, printed, or downloaded as PDFs.
Get started now—even if you don't have a collection kit
- Download the Test Requisition Form (TRF)
- Select “Exact Managed” as the sample collection method
- Fax or email the completed TRF to Exact Sciences
- Kits are shipped directly to your patient, who will receive a notification to self-schedule their blood draw
Test Requisition form
Blood collection kits
Have questions?
Footnotes and references
- Medical Science Liaisons
- Exact Sciences. Cancerguard™ Test Information for Healthcare Providers ; Published 2025. Accessed July 22, 2025.
- Exact Sciences. Cancerguard™ Care Navigation Guide; Published 2025. Accessed July 23, 2025.
Important information about the Cancerguard test
Rx only. The Cancerguard™ test is indicated for use in adults ages 50-84 with no known cancer diagnosis in the last three years. It detects alterations in circulating tumor DNA and tumor-associated protein levels which are commonly associated with cancer. The test does not detect all cancers and is not a replacement for existing recommended cancer screening or diagnostic modalities for cancer. Current recommended screening for cancer should also be followed. The test is not indicated for screening of breast and prostate cancer and was not evaluated for the detection of pre-cancerous lesions. Due to the potential for follow-up imaging with IV contrast CT after a positive test result, careful consideration should be given before ordering the Cancerguard test for patients with a history of adverse reactions to iodine based IV contrast or for women who are, may be, or plan to become pregnant. Results should be interpreted in the context of a patient’s medical history, clinical signs, and symptoms. A negative result does not rule out the presence of cancer of any type. A positive Cancerguard™ test result means that the blood test identified a cancer signal that may indicate the presence of cancer. This result alone does not confirm the presence of cancer. Further clinical evaluation by a healthcare provider (which may include blood tests such as complete blood count and comprehensive metabolic panel) and follow-up imaging are needed to locate and confirm a diagnosis of cancer or determine that cancer is not present. While there are no established guidelines for imaging following a positive Cancerguard test result, there is a published follow-up workflow based on expert clinician opinion and results from an exploratory, prospective, interventional study [Kisiel et al Life (Basel) 2024 Jul 24; Lennon et al, Science, 2020]. The proposed workflow in the Kisiel et al publication includes an intravenous-contrast enhanced computed tomography (IV contrast CT of chest, abdomen/pelvis, and soft-tissue neck) and if necessary, positron emission tomography-computed tomography (18F FDG PET-CT) from the skull-base to mid-thigh. Alternative follow-up procedures (e.g. targeted imaging, endoscopic procedures, CT without IV- contrast) may be appropriate in the context of the patient’s medical history and clinical evaluation [Lennon et al, Science, 2020]. False positive and false negative test results can occur.
The Cancerguard test was developed, and the performance characteristics validated by Exact Sciences Laboratories following College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA) regulations. This test has not been cleared or approved by the US Food and Drug Administration. The test is performed at Exact Sciences Laboratories. Exact Sciences Laboratories is accredited by CAP, certified under CLIA regulations, and qualified to perform high-complexity clinical laboratory testing.