Continued Momentum Through the Second Quarter

Exact Sciences recently announced 2017 second quarter earnings results, which exceeded our expectations. Revenue reached $57.6 million, up 19% sequentially, and completed Cologuard test volume totaled 135,000, up 35% sequentially.
Second quarter cost of sales totaled $133 per completed Cologuard test, down from $170 in the first quarter and significantly better than we expected. During the quarter, we saw volume leverage and efficiencies in our lab and manufacturing operations and benefited from a previously disclosed royalty buyout agreement.
We believe the underlying improvement in cost of sales per test is sustainable over the long term. However, we expect scale-up investment in infrastructure and personnel will lead to a temporary increase in cost per test starting in the third quarter.
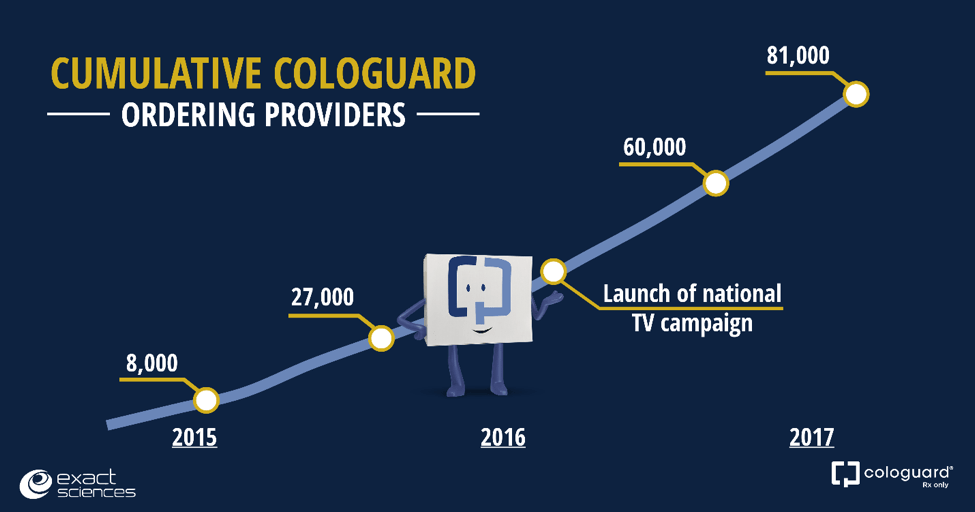
Since Cologuard launched in 2014, 81,000 physicians and other healthcare providers have ordered the non-invasive colon cancer screening test. More than 800 healthcare providers a week ordered Cologuard for the first time during the second quarter. As Kevin Conroy, our chairman and CEO, highlighted on yesterday’s call, “We are changing the paradigm for colon cancer screening.”
Our national television campaign has been remarkably effective in creating patient demand and driving new physician adoption at a consistent pace. Approximately 50,000 primary care physicians of the more than 200,000 we are targeting in the United States have ordered a Cologuard test. There are still many physicians and people ages 50 to 85 to reach.
We believe that Cologuard’s estimated 86% insurance coverage will help increase adoption among those physicians. The number of total lives covered by healthcare insurers for Cologuard increased to 235 million as of July 25, 2017. Our market access team has been focusing on contracting to allow patients access to Cologuard as an in-network benefit.
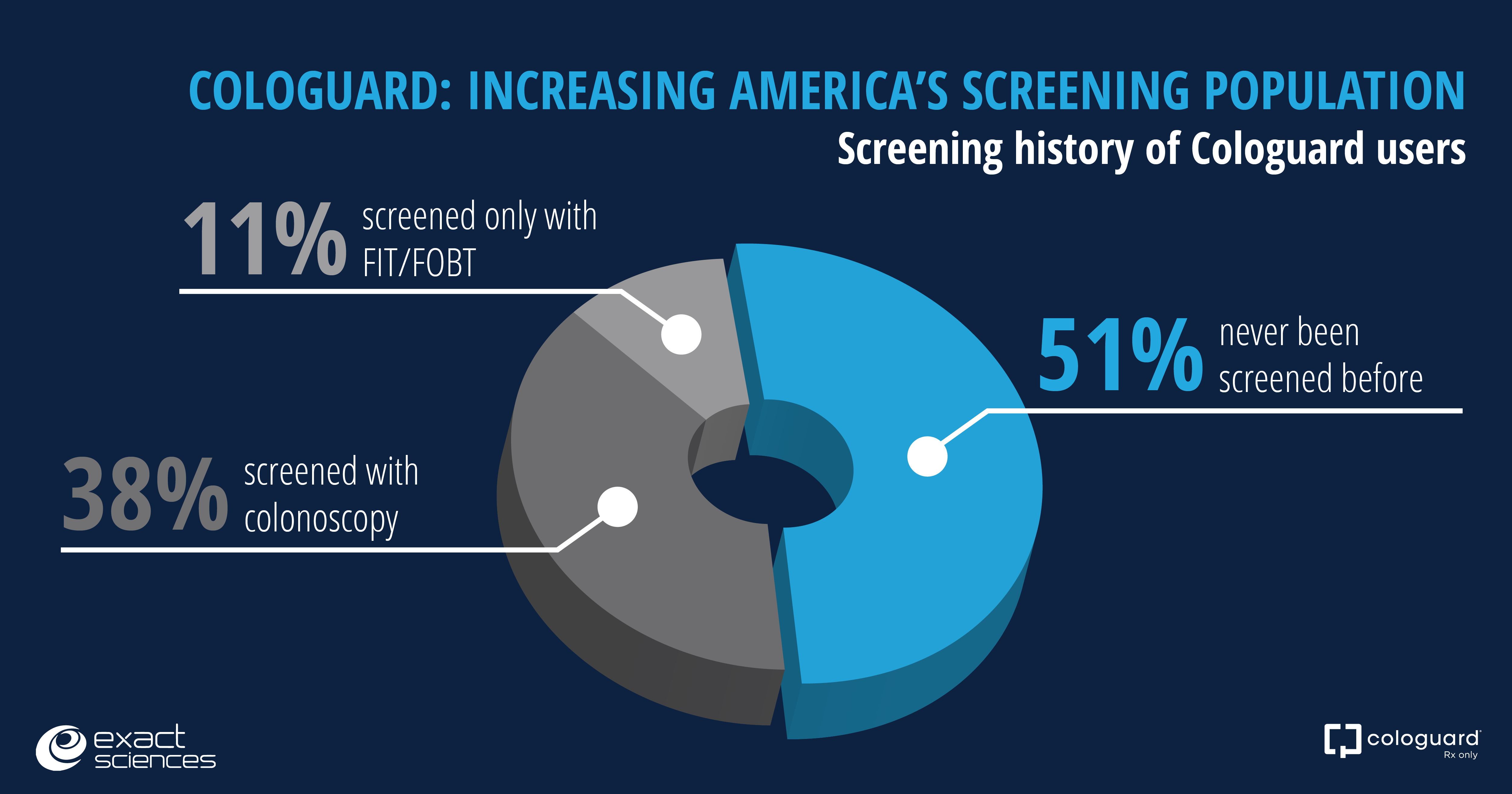
More than half of Cologuard users screened for first time
New data from the second quarter show that more than half of Cologuard users reported never before being screened for colorectal cancer. These data demonstrate that Cologuard is ensuring more people get screened for colon cancer. Many Cologuard users are in their early fifties, which gives us the potential to continue screening these patients for years to come.
2017 guidance
Exact Sciences had a strong second quarter, and we are pleased to raise our full-year revenue guidance. We now expect revenue of $230 to $240 million in 2017, and Cologuard volume of at least 550,000 completed tests. For the third quarter, we expect Cologuard volume of at least 150,000 completed tests.
Investing in long-term, sustainable growth
Looking to Cologuard’s future, we are investing in people and infrastructure to support our continuing growth. As Kevin highlighted, “We recognize that reaching our goal of eradicating colon cancer will take time and require further investments, hard work, and intense focus from the entire Exact Sciences team.”
Exact Sciences is one of few companies in cancer diagnostics to possess the human and financial resources to make a major impact on a significant number of lives. Exact Sciences uses a differentiated, low-cost, highly accurate, proprietary platform. This platform, coupled with our capabilities, will enable us to bring advanced accurate tests to people who need them in a highly cost-effective way, providing a long-term sustainable advantage.
The company's guidance for revenue and completed tests are forward-looking statements. They are subject to various risks and uncertainties that could cause the company's actual results to differ materially from the anticipated targets. The anticipated targets are not predictions of the company's actual performance. See the cautionary information about forward-looking statements in the "Safe Harbor Statement" section of this press release.
About Cologuard
Cologuard was approved by the FDA in August 2014 and results from Exact Sciences' prospective 90-site, point-in-time, 10,000-patient pivotal trial were published in the New England Journal of Medicine in March 2014. Cologuard is included in the American Cancer Society's (2014) colorectal cancer screening guidelines and the recommendations of the U.S. Preventive Services Task Force (2016) and National Comprehensive Cancer Network (2016). Cologuard is indicated to screen adults of either sex, 50 years or older, who are at average risk for colorectal cancer. Cologuard is not for everyone and is not a replacement for diagnostic colonoscopy or surveillance colonoscopy in high-risk individuals. False positives and false negatives do occur. Any positive test result should be followed by a diagnostic colonoscopy. Following a negative result, patients should continue participating in a screening program at an interval and with a method appropriate for the individual patient. Cologuard performance when used for repeat testing has not been evaluated or established. For more information about Cologuard, visit www.cologuardtest.com. Rx Only.
Safe Harbor Statement
This news release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that are intended to be covered by the "safe harbor" created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as "believe," "expect," "may," "will," "should," "could," "seek," "intend," "plan," "goal," "estimate," "anticipate" or other comparable terms. All statements other than statements of historical facts included in this news release regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding expected future operating results, anticipated results of our sales and marketing efforts, expectations concerning payer reimbursement and the anticipated results of our product development efforts. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to successfully and profitably market our products and services; the acceptance of our products and services by patients and healthcare providers; our ability to meet demand for our products and services; the willingness of health insurance companies and other payers to cover Cologuard and reimburse us for our performance of the Cologuard test; the amount and nature of competition from other cancer screening products and services; the effects of the adoption, modification or repeal of any healthcare reform law, rule, order, interpretation or policy; the effects of changes in healthcare pricing, coverage and reimbursement; recommendations, guidelines and quality metrics issued by various organizations such as the U.S. Preventive Services Task Force, the American Cancer Society, and the National Committee for Quality Assurance regarding cancer screening or our products and services; our ability to successfully develop new products and services; our success establishing and maintaining collaborative, licensing and supplier arrangements; our ability to maintain regulatory approvals and comply with applicable regulations; and the other risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our most recently filed Annual Report on Form 10-K and our subsequently filed Quarterly Report(s) on Form 10-Q. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.